Structure of Water for Origin of Life and Living Matter
Prof. Dr. Ignat Ignatov
Ass. Prof. Oleg Mosin PhD
Additional information about the report:
www.medicalbiophysics.dir.bg/en/water_memory.html
1. Living organisms are complex self-organizing systems
Living organisms and man are complex self-organizing systems. They are open, because they continually exchange substances and energy with the environment. The changes in the open systems are relatively stable in time. The stable correlation between the components in an open system is called a dissipative structure (Nikolis, Prigozhin, 1973). It has been proven experimentally that water is also a self-organizing system (Antonov, Galabova, 1992). The expectations are that changes in water as a result of an external influence will be relatively stable in time. This signifies that water remembers
physical or chemical influences. The question about water memory
is of exceptional interest. The first research studies, related to the memory of water, were effected by Deryagin and Churaev (1971). The durability of the results in time after an activation
with an alternating magnetic field and upon electrolysis through a nuclear filter were performed by Minenko (1981) and Evseev (1982). Analyses have been carried out on the changes in the spectrum of natural waters (Antonov and co-authors, 1995).
The question of how long information is stored by the water molecules is controversial in modern science. On the other hand, water has a number of unique properties that allow it to store and disseminate information as a result of the external physical or chemical factor of influence. In a physical sense the correct term is informationability
of water (Ignatov). Yet one can hardly explain the origination of living matter without this property (Ignatov, Mosin).
According to the originator of quantum mechanics, Schrodinger, living organisms decrease their own entropy at the expense of the increase of environmental entropy. According to Prigozhin, the formation of dissipative structures and their complication is related to changes in entropy.
The origination of a living cell is possible under extreme conditions and, with time, the stabilization of said conditions, which preserves the originating structures. The formed self-organizing structure aims to safeguard its state from the conditions of the external environment. This calls for the universal solvent –water – with its unique properties and specific substances. The vitality of living matter is proved even in the existence of archi-bacteria. They live in conditions of strong radioactivity, low temperatures, in volcano craters.
It looks like the water drop evaporates gradually. Antonov and Yuskeselieva prove a new physical effect. The water drop evaporates discreetly (unevenly). This effect depends on the energy states of the hydrogen bonds between the oxygen atoms of the water molecules and the hydrogen atoms of neighboring molecules.
Antonov and Galabova prove through spectral analysis that water is an open and self-organizing system. Water and the living organisms react sensitively to energy flows and store information from the environment. They apply the method of the Differential Non-equilibrium Energy Spectrum (DNES).
The water molecule clusters are the smallest and most unstable self-organizing structures in Nature (Ignatov, 2005). Alterations in the water clusters as a result of internal influences could be relatively stable in time. The larger the cluster formation, the longer time information is stored regarding the physical or chemical factor. Stable self-organizing structures are obtained, which could carry future information concerning living matter.
Water molecules are restructured as a result of external influences. When they have received energy, they transfer information about their status to neighboring molecules through hydrogen bonds. This is done on the resonance principle (Zenin, 2002 ), (Ignatov, 2005).
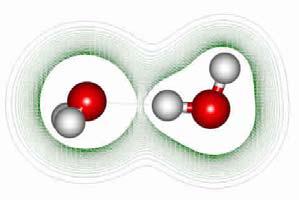
Hydrogen bond among water molecules Clusters of water molecules are structured with the hydrogen bonds
In order to explain the structuring of clusters, one should approach it from a quantum-mechanic way. In the contrary case, one would find it hard to explain how the water molecules are structured in geometric clusters (polymers
).
The classic polymer – this is a molecule in which the atoms are connected through covalent bonds, not through hydrogen ones. It is experimentally proven, that 10% of the hydrogen bonds inice are covalent (Isaac, 2002).
Emoto managed to visualize the arrangement of the water molecules in clusters by freezing the water after different types of influences. The crystals of frozen water are arranged in a certain way. The ice remembers
the pre-history of the liquid water phase after an influence has been effected. He conducted an experiment by influencing water with different frequencies. The interesting thing is that the higher the frequency, the clearer the pictures. This is a visible proof that water reacts differently to different influences (Emoto, 2002).

With the origination and evolution of living matter, the water molecules, the cells and the tissues exchange biophysical fields among them (Ignatov, 1998). Depending on the energy status, the energy from the environment is either received in the living organisms (give
mode) or is lost (take
mode) (Dr. Ignatov, Antonov,Galabova, 1998). The same authors prove that ingive
and take
mode an energy redistribution is effected among water molecules.
2. Homeopathy does not give grounds for the making of fundamental conclusions regarding information abilities
of water
There exist interesting proof already that several molecules from a certain substance can significantly change the structure of water clusters. Homeopathy is a classical example for this. Experiments were conducted under the leadership of the French immunologist Benveniste. The homeopathic principle was reproduced on biological models. When to one of the immune cells types in man specific antibodies interacting with them were added, a cell reaction was observed. Upon decrease of the concentration of the antibodies in some dilutions, an effect was observed, and in other cases – it disappeared. Such an alteration of biological activity of solutions
was also observed in concentrations when the probability of the presence of even one protein molecule was only slight. The authors presumed that the transfer of biological information was due to the memory
of water. Double blind
experiments with homeopathic solutions of Benveniste do not prove the initially obtained positive results. Experiments have been conducted with inorganic substances,which were diluted according to the homeopathic principle. Ray decided to disprove the homeopathic views. He diluted sodium chloride ( NaCl ) and lithium chloride ( LiCl ) to a minus tenth degree. He also researched water with thermo-luminescence. The strange thing was that the researched solutions had a different spectrum. Research has been effected concerning the alterations in the spectrum of homeopathic solutions from 1 to 15 potencies (solutions
) (Ignatov, 2005). The research was conducted through the DNES method of natrium muraticum (NaCl) . At 1 CH the solution has 0.01 %, and at 2 CH it contains 0.0001% of NaCl, etc. In this process the solution passes the Avogadro number. After this value it is accepted that no molecules of the diluted substance are present.
Analyses show that up to 6 CH, the changes in the homeopathic solutions are close to the result at 1 CH. At 5 CH the concentration of the solution is 10-10 for NaCl, as in Ray’s experiments. After 11 CH, the result is close to or is in the framework of the statistical error.
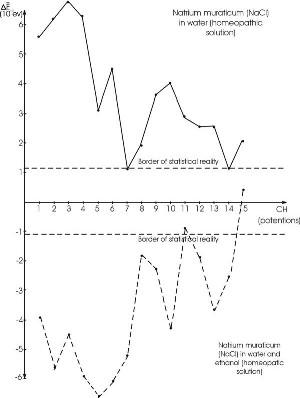
Dependence between average hydrogen bonds energy in homeopathic solution and its potencies (dilutions). (Ignatov, 2005)
The larger number of molecules of NaCl in the initial potencies creates conditions for stability of the clusters formed from the initial substance and the water molecules. The potency process transfers
information to the larger potencies as well. This information is more unstable
, when, subsequently, it is transferred by water molecules only.
Studies of the properties of homeopathic solutions have one peculiarity. In the homeopathic solution the effect is influenced not only by the diluted substance and the potentiation, but also by a third feature that researchers do not report. The solution itself is potentiated in an electromagnetic device and electromagnetic fields indicate to the device an influence on the hydrogen bonds between water molecules. This means that this method of preparation of homeopathic solutions can not serve for the making of fundamental conclusions about informational properties of water.
3. Water, carbon, elements for the origination of life and living matter
There is indisputable proof that water lies in the foundation of living matter in the favorable conditions of our planet. Without the property of water informationability
, one could hardly explain the origination of living matter. The clusters, which interact with chemical substances, effect evolution. The element that has played a decisive role is carbon (C). The small size of the atom allows for the carbon chains to bend. An indicator for the organic origin of carbon on Earth is the change of the ratio between its isotopes 12С and 13С for 3,8 billion years.
Yet what is the probability for a formation of water clusters, micro elements and additional conditions to create organic molecules, and, subsequently, living cells. Lets look at the smallest living cells. The smallest bacterium Micrococcus progrediens is 0.1 µm in diameter. The myco plasma is also 0.1 µm. This means that these cells are 1000 larger in diameter than the hydrogen atom. The myco plasma has the requisite macro molecules for the existence of a living cell. The interesting thing for it is that it has a flexible membrane, not a solid one, like the other cells. The myco plasma reproduces through the formation of coccus-like structures or division. The electric properties of the membrane, which is 0.1 µm wide, do not differ from those of the remaining cells.
The structure of clusters of water molecules with dimensions 1,1 µm / 1,1 µm / 203 Å is stable regarding the effectuation of biological processes. (Zenin, 2002).
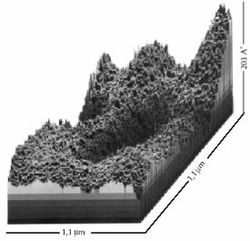
memorization
of water.
The presence of the elements carbon (C), calcium (Ca), magnesium (Mg), sodium (Na), etc., could lead to the formation of cluster structures that are more stable in time. The stability of these clusters depends on the interaction between the separate molecules. The availability of carbon compounds and ions of chemical elements could lead to an uncompensated electric charge and potential in a structure that is stable for the effectuation of biological processes. The proof for the part played by calcium carbonate upon origination of living matter (Dr. Ignatov, Antonov, Galabova, Stoyanov, 2001) is that the most ancient photosynthesizing organisms have a complex layer-like structure from this compound. The first evidences for the presence of water date from this stage.
The conduct of an electric bio-current and the structuring of the cellular membrane has made possible the transition from non-living to living matter. Additional experiments are required for the clarification of this process. Erythrocytes consist predominately of a cellular membrane, and blood contains 92% water. Life on Earth has an unified origin, because each living cell has 20 amino acids, 2 carbohydrates and 1 phosphate.
Under certain conditions one could also speak of activated
water. Yet this statement brings suspicions in some scientists. It is proven that cancer cells lacerate
the energy hydrogen bonds among water molecules the most (Antonov, Galabova, 1992). The blood plasma of person who has died from a malignant tumor does not react to biophysical fields (Tushmalova, 2001). Yes, water can be activated and bear information regarding the living. One of Emoto’s experiments shows crystallized water after a strong earthquake and 3 months later. A conclusion can be drawn from this experiment too that, under favorable conditions,water can concentrate information in a small volume of water clusters.
4. Emoto’s snowflakes demonstrate, but do not prove
Emoto’s crystals provoke both an interest and an insufficiently grounded criticism. If we look at them more carefully, we will see that they are structured with six apexes. Yet an analysis that is even more detailed shows that winter snowflakes are also always symmetrical and have six branches. To what extent do these crystallized structures carry information about the environment they have been created in? Snowflakes can be beautifully structured and formless. This shows that the control sample
(a cloud in the atmosphere) from which they originate influences them with the initial conditions. The initial conditions are solar activity, temperature, geophysical fields, humidity, etc. This means that a conclusion can be made for the approximately similar structure of the water drops and afterwards snowflakes from an average
ensemble. They have an almost identical mass and move in the atmosphere with a close speed. In the atmosphere they continue to structure themselves and to increase their volume. Even if they have originated at a different place in the cloud, there is a sufficient number of snowflakes that have originated in almost identical conditions.
We can find an answer to the question what is a positive
and negative
information about snowflakes with Emoto. In laboratory conditions, negative
information (earthquake, bad
acoustic vibrations for man, etc.) does not structure crystals. But positive
information does. Yet it is interesting to what extent one factor can structure identical or similar snowflakes. Water is at its most dense at a temperature of 4°C. It has been scientifically proven that its density decreases with the initiation of formation of hexagonal ice crystals upon decrease of temperature below 0°C. This is due to the hydrogen bonds among the water molecules.
But what is the reason for this structuring. Crystals are solid bodies whose constituent atoms, molecules or ions are arranged in a regular, repetitive structure in the three spatial dimensions. In water the crystal structure is slightly different. According to Isaac, only 10% of the hydrogen bonds in ice are covalent, i.e., sufficiently informationally stable. The hydrogen bonds between the oxygen of one water molecule and the hydrogen of another are most sensitive to external influences. The water spectrum in the cluster arrangement is relatively different in time. According to the effect of discreet evaporation of the water drop proven by Antonov and Yuskesselieva and the dependency of this effect on the energy states of the hydrogen bonds, we can search for an answer regarding the structuring of crystals. Each part of the spectrum depends on the surface tension of the water drops. The piques in the spectrum are six and informationally they direct
the branches of the snowflake (Dr. Ignatov, 2009).
Apparently, in Emoto’s experiments, the initial control
sample influences the crystal type. This means that after an influence with a specific factor, similar crystals could be expected. It is almost improbable to receive identical crystals. With the example for the influence of the word love
on water, Emoto has not indicated clearly whether the experiment has been conducted from different samples.
Double blind
experiments are required to check whether Emoto’s methodic is sufficiently differentiated. Isaac’s proof that 10% of the water molecules form covalent bonds after freezing shows us that probably water uses this information potential upon freezing. The achievement of Emoto even without double blind
experiments remains sufficiently significant with regard to water’s information abilities.
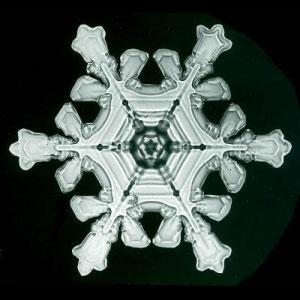
Natural snowflake
Wilson Bentley, 1925

Snowflake of Emoto,
formed by natural water
One of the snowflake is natural, the other has been created by Emoto. The similarity indicates that the variety in the water spectrum is not infinite. Research has been made of the spectrum of natural waters from Teteven, Troyan, Rila Monastery (Bulgaria), Gersfeld (Germany). The spectrum is highly energy-charged at the strongest hydrogen bonds. With deionized water such a characteristic is missing. The cancer cells tear
most highly the energy bonds in water. This means that natural
waters carry the requisite information for living systems (Ignatov, 2009).
On November 15, 2008, an earthquake with a magnitude of 4.0 on the Richter scale was registered with an epicenter in Sofia.
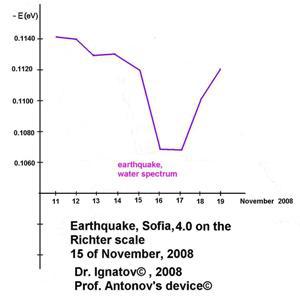
Earthquake, Sofia,
4.0 Richter scale, 15 November 2008,
Dr. Ignatov, 2008©, Prof. Antonov's device©
The figure shows the difference between the control sample and the ones from the remaining days. The water molecules tear
most highly the energy hydrogen bonds in water, as well as two piques in the spectrum during the natural phenomenon. The research was made with Antonov’s device (Ignatov, 2008). During an earthquake, water cannot be structured into snowflakes in Emoto’s laboratory. There is evidence about a change in the electric conductivity of water during an earthquake.
In 1963, the Tanzanian high school student Erasto Mpemba observed that hot water freezes faster than cold water. This phenomenon is called the Mpemba effect
. Actually, much earlier than Mpemba, this unique water property was noted by Aristotle, Francis Bacon, and Rene Descartes. This phenomenon has been proven with a series of independent experiments. Water demonstrates yet another one of its strange properties. In my opinion, the explanation is the following: the differential non-equilibrium energy spectrum (DNES) of boiled water has a smaller average energy of the hydrogen bonds among the water molecules than the sample having a room temperature (Ignatov, Antonov, Galabova, 1998). The boiled sample needs less energy to begin to structure crystals and to freeze (Dr. Ignatov, 2009).
To what extend can Emoto’s experiments be related to the “informationability of water. His experiments prove that water reacts to external influences. In his laboratory snowflakes lack the natural variety. They are formed in more standardized conditions. The valuable thing is that he demonstrates that water “remembers” external influences. The question regarding the longevity of this information in his experiments remains open.
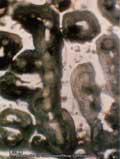

A B
Crystallized water after a strong earthquake (A) and 3 months later (B), Emoto.
5. Defrosted water. Water structures as DNA in nanotubes
Defrosted water is exceptionally active
. In spring, an increase of the amplitudes in the spectrum of water, measured through the DNES method is observed. In this season birds and animals drink water from defrosted ice. Plants also grow quickly from this water. Defrosted water has a stimulating effect and is used successfully for the convalescence of diseased people after an operation. Scientists from the Royal Medical Institute in Stockholm report of a successful treatment of diabetes with defrosted water. The healing properties of defrosted water are due not only to the more active
spectrum. Even more active is the protonated water. Deuterium is practically cleaned out by freezing a small part of the water. The layer of ice contains the deuterium and it is removed. The remaining drinking water is protonated, i.e. deuterium-free water. Similar properties can be found in mountain water in which the process of cleaning out the deuterium is effected during the passage of water through earth layers. The water molecules in which the hydrogen atoms are from the deuterium isotope have difficulty passing through the cellular membrane. Marinov reported of a quick growth of flowers in Siberia. Together with Russian scientists in the 70ies, he proved that the water in the region contained less deuterium.
In the 19th century Pasteur noted that in inanimate nature molecules are symmetrical. In animate nature molecules are mirror image asymmetrical. Proteins are made up of left-oriented amino acids. This property is determined by the rotation of the plane of polarization of light from the molecule. How to explain this phenomenon?
The presence of asymmetry in organic molecules could have been obtained when an open system, which precedes the biosphere, has been in extremely critical non-equilibrium state. Evolutionary transition was effected with a leap, which is typical for self-organization.
An example of this condition are the experiments in which water molecules resemble DNA in nanotubes. American scientists led by Xiao Chen Shen managed to make an interesting experiment. At high pressure and low temperature water molecules of ice form structures resembling the double helix of DNA. Under these extreme conditions hydrogen bonds are bent.
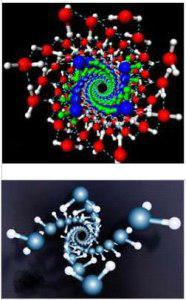
Water structures as DNA in nanotubes,
Xiao Chen Shen et al, New Scientist, USA
A transition from symmetric molecules of inanimate nature to asymmetric biomolecules of animate nature may have occurred in the early stages of chemical evolution as self-organization of matter. Antonov demonstrates that water is also an open system and shares energy and substances with the environment (Antonov, 1992).
A team of researchers has conducted a very interesting experiment in the Arctic. A drilling was done in the ice at a depth of half a kilometer. Layers of ice from different years were clearly visible. An isotopic analysis was made of the deuterium and of oxygen isotopes. Water has always been able to remember
information from the respective year. It turned out that the coldest were the XV, the end of the XVII and the beginning of the XIX century. The warmest were 1550 and 1930.
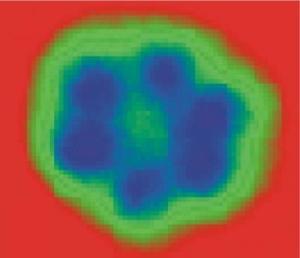
Cluster formation of 6 water molecules, Michaelides, Morgenstern, Nature
Michaelides from the Nanotechnology Center in London and Morgenstern from the Leibniz University in Hannover have published in the Nature journal results of studies of the nano-level of water. Photography shows the smallest connected formation of 6 water molecules in ice.
6. Structure of water
The effect of Antonov and Yuskeselieva of 1983 showed that the water droplet evaporates discretely (in a salutatory fashion). This effect depends on the energy states of hydrogen bonds between the oxygen atoms of water molecules and the hydrogen atoms of the neighboring molecules. Water molecules themselves are linked into clusters. One of the evidences is the clusters of water molecules sized 1,1 μm / 1,1 μm / 203 Å, photographed by Zenin with an electronic microscope. The obtained result has not been replicated in other laboratories, so it is difficult to be accepted as scientific foundation yet. The very structures of Zenin’s photography are about 40 nanometers in size. A team of Japanese scientists headed by Naguib published information that small clusters of water molecules and carbon have been observed with an electronic microscope. Their size was 20 to 50 nanometers.
(1 nanometer=10-9 meters)
The smallest bacterium Micrococcus progrediens is sized 0.1 μm or 100 nanometers in diameter. This means that self-organization and structuring of living matter may begin within the stable cluster formations.

Result of Geissler, Saykally and Smith with Raman spectroscopy upon analysis of the movement of water molecules,
Berkeley University, USA
In 2005 a team from Berkeley University, USA – Geissler, Saykally and Smith, demonstrated with Raman spectroscopy that hydrogen bonds among water molecules are constantly tearing, changing and moving. These results correlate with my quantum-mechanical analysis of water spectrum. In these analyses the relative stability of clusters depends on external factors. Water is different in its structure, and a similarity in the spectrum can be observed in the presence of certain external factors (Ignatov, Mosin, 2005). Water changes the position of water molecules depending on the energy of hydrogen bonds. The apparatus results and analyses for the presence of stationary
clusters can hardly be accepted. Cluster formations themselves are dynamic and the memorization
of information depends on a series of factors. The first results and analyses with Antonov’s device were obtained in 1997.
The chart above shows the results of the PNAS, USA study on the possible number of hydrogen bonds depending on the number of water molecules. Upon an increase of the number of hydrogen bonds, the nano-droplets stability is reduced. This result correlates with my quantum-mechanical analysis regarding dynamic movement of water molecules in their attempt to find a relatively stable condition of clusters of the order of nanometers.
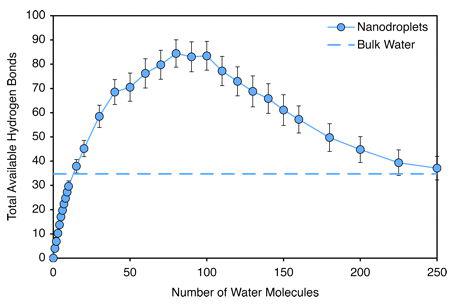
Proceedings of the National Academy of Sciences (PNAS), USA
Let us translate the results from the chart into the language
of quantum physics and biophysics. When water droplets evaporate, the spectrum of the hydrogen bonds with the lowest energy, around -0.09 to -0.1 eV is obtained in the beginning. These water molecules may be linked in clusters, and may also be free. At -0.11 eV a peak is observed, which (this is proven experimentally) is related to the presence of calcium ions in the water (Antonov, Galabova, 1992). The two authors have studied a solution of calcium carbonate and water from the Temnata Dupka
cave, Bulgaria. The results correlate at level of p<0.05. This is part of a cluster formation. With the increase of the energy of the hydrogen bonds among the water molecules to -0.14 еV, the cluster formation completes its structuring. A redistribution according to energies between the water molecules is observed (Ignatov, Antonov, Galabova, 1998).
In the presence of trace elements and compounds, clusters are more stable. In the control deionized
water measurements show that not many stable clusters are formed.
Studies with the device of Antonov of the spectrum of water that interacts with living tissue show an increase in the parameters of the spectrum between -0.1362 eV to -0.1387 eV. Cancerous tissue in this part of the spectrum decreased these parameters. One cluster formation begins
to self-organize when it seeks to preserve
the stronger energy interaction among the molecules in it. We can define energy as conservation
of self-organization
of stronger energy levels in clusters of molecules (Ignatov, Mosin, 2010).
Chaplin defines cluster structures in which the carbon atoms are in the cluster nucleus. His evidence correlates with my examining of the difference between the qualities of water with regard to the creation of cluster formation, which carry information about the living matter. Chaplin considers the structure C60(H2O)80
Chaplin's results correlate with the Hofmeister series. These series are related to the classification of ions in terms of their ability to change structures in water. The measurements with Antonov’s device also correlate with the Hofmeister series.
The results of Chaplin also correlate with the measurements of Andrievsky with piezo gravitometry for (20 - 24 H2O for C60). Similar results were obtained with colorimetry. The number of water molecules at 0 degrees is 60 H2O for C60.
In depleted
water it is about 20 water molecules. These results and analyses of Chaplin are fundamental to the fact that different types of water have a number of water molecules in terms of their binding with carbon molecules. The amplitudes of the spectrum of depleted
water are the lowest. The spectrum of the depleted
water is close to the spectrum of deionized
water. The amplitudes in natural waters are high, especially in the hydrogen bonds of strongest energy of -0.1362 еV to -0.1387 еV.
7. Hot mineral water for origin of life and living matter
In late 2009 and early 2010 Ignatov and Mosin carried out experiments with control deionized
water, mineral water, sea water, and mountain water from Bulgaria. Water from karst springs was also studied. The experiments were made with Antonov’s device for spectral analysis of water. Cactus juice was studied too (Ignatov, Mosin, 2009). The cactus was selected as a model system because the plant contains about 90% water. Also, photosynthesis is carried out by the enlarged stems, which serve for storage of water as well. Mineral water from different springs was examined.
A proof of this is that the oldest traces of photosynthesizing organisms are the stromatolites. The oldest stromatolites were found in Greenland. They are 3.5 billion years old. They have a complex laminar structure of calcium carbonate and extract hydrogen from water. Even today they exist in Shark-Bay, Australia. Perhaps there has been mineral water at their very inception at the bottom of the water basins, or after their creation close to the shore, they have spread in water basins. Life has been discovered at the bottom of the oceans under extremely harsh conditions and it was near hot mineral springs.
The plant cell cannot exist without the following organogenic elements C, H, N, P, O, S and the elements Na, K, Ca, Mg, Cl, B, etc.
Let’s review the following reactions:
(1) CO2 + 4H2S + O2 = CH2O + 4S + 3H2O
(2) СаСО3+ HOH + СО2 = Ca(HCО3)2
The first equation shows how some chemosynthetic bacteria use energy from the oxidation of hydrogen sulfide (H2S) to sulfur (S).
The second equation is related to one of the most common processes in nature.
In the presence of water and carbon dioxide, calcium carbonate transforms into calcium hydrogencarbonate.
In the presence of hydroxyl OH- ions, the cellular processes are activated. Kagava demonstrates that an effect of improving the conductivity of the cell membrane is observed. The valid reaction is:
(3) CO2 + ОН- = HCО3-
(4) 2 HCO3- + Ca2+ = CaCO3 + CO2 + H2O
It is assumed that the second reaction has been valid upon the origination of the stromatolites.
Contemporary chlorophyll contains the elements C, H, O, N, Mg.
Bibliography:
- Fox S.W., Krampitz G. (1964) Catalytic decomposition of glucose in aqueous solution by thermal proteinoids, Nature, Vol. 203, pp. 1362–1364.
- Fox C.W., Wang C.T. (1968) Melanocytestimulating hormone: Activity in thermal polymers of alpha-ammo acids, Science, Vol. 160, pp. 547–548.
- Sugawara T. et al. (2011) Self-reproduction of supramolecular giant vesicles combined with the amplification of encapsulated DNA, Nature Chemistry, Vol. 1127, pp. P. 775–780.
- Ward D. (2010) First Fossil-Makers in Hot Water, Astrobiology magazine, №1.
- Pons M.L. (2011) Early Archean serpentine mud volcanoes at Isua, Greenland, as a niche for early life, Proc. US National Acad. Sciences, Vol. 108, pp. 17639–17643.
- Mulkidjanian, A. Y.; Galperin, M. Y. (2009) 1. On the origin of life in the Zinc world. 2. Validation of the hypothesis on the photosynthesizing zinc sulfide edifices as cradles of life on Earth, Biology Direct.
- Ignatov, I. (2010) Which Water is Optimal for the Origin (Generation) of Life? Euromedica, Hanover, pp 34-37.
- Ignatov, I. (2011) Entropy and Time in Living Organisms, ArchivEuromedica, Hanover, 1st&2nd Edition, pp. 74-75.
- Informationability of Water, Kirlian (Electric Images) of Different Types of Water, Euromedica, Hanover, pp. 62-65.
- Ignatov, I. (2012) Origin of Life and Living Matter in Hot Mineral Water, Conference on the Physics, Chemistry and Biology of Water, Vermont Photonics, USA.
- Ignatov, I., Mosin, O. V (2012) Isotopic Composition of Water and its Temperature in Modeling of Primordial Hydrosphere Experiments, Euro-Eco, Hanover, p. 62.
- Ignatov, I., Mosin, O. V (2012) Isotopic Composition of Water and its Temperature in Modeling of Primordial Hydrosphere Experiments, VIII Int. Conference Perspectives of the Development of Science and Technique, Biochemistry and Biophysics, Vol. 15, pp. 41-49.
- Ignatov, I., Mosin, O. V (2013) Isotopic Composition of Water and its Temperature in Modeling of Primordial Hydrosphere Experiments, Science Review, №1, pp. 17-27.
- Игнатов, И., Мосин, О. В. (2013) Изотопный состав воды и ее температура в процессе эволюционного происхождения жизни и живой материи, Науковедение, Т. 14, №1, С. 1-16.
- Мосин, О. В., Игнатов, И. Изотопные эффекты дейтерия в клетках бактерий и микроводорослей при росте на тяжелой воде (D2O) //Вода: химия и экология. 2012 № 3. С. 83-94.
- Мосин, О. В., Игнатов, И. Изучение изотопных эфектов тяжелой воды (D2O) в биологических системах на примере клеток прокариот и эукариот//Биомедицина. 2012. Т.1. №1-3. С. 31-50.
- Мосин, О. В., В.И. Швец, Складнев Д.А., И. Игнатов. Микробный синтез дейтерий-меченного L-фенилаланина факультативной метилотрофной бактерией Brevibacterium Methylicum на средах с различными концентрациями тяжелой воде// Биофармацевтический журнал. 2012. Т.4. №1. С. 11-22.
- Мосин, О. В., Игнатов, И. (2012), Загадки ледяных кристаллов, Сознание и физическая реальность, Т. 17, No. 5, С. 21-31.
- Mosin, O. V, Ignatov, I. (2012) Kirlian Effect in Biomedicine Diagnostics and Research of Properties of Biological Objects and water, Biomedical Radio electronics, Biomedical Technologies and Radio electronics, pp. 13-21.
- Mosin, O. V, Ignatov, I. (2012) Structure of Water, Chemistry, Moscow, No. 11, pp. 24-27.
- Gulyaev, Yu. V., Godik, E. E (1990) Physical Field of Biological Objects, Scientific American, №5, p. 75.
- Gulyaev, Yu. V., Godik, E. E. (1983) Physical Field of Biological Objects, Newspaper of USSR Academy of Science. SU, №8, p. 118.
- Ignatov, I. (2005) Energy Biomedicine, Gea-Libris, Sofia.
- Мосин, О. В., Игнатов, И. (2011) Осознание воды как субстанции жизни, Сознание и физическая реальность, Т. 16, No. 12, С. 9-21.
- Игнатов, И., Мосин, О. В. , Изотопный состав воды и долголетие, Науковедение, Т. 14, №1, С. 2-10.
- Игнатов, И., Мосин, О. В., Нанева, К. (2012) Вода в человеческом теле несет информацию о долголетии, Naturopathie, С. 39-41.
- Мосин, О. В., Игнатов, И. (2011) Структура воды и физическая реальность, Сознание и физическая реальность, Т. 16, No. 9, С. 16-31.
- Мосин, О. В., Игнатов, И. (2012) Адаптация к тяжелой воде. Фенотипическое или генетическое явление? Сознание и физическая реальность, Т. 17, No. 4, С. 25-36.
- Мосин, О. В., Игнатов, И. (2011) Разделение тяжелых изотопов дейтерия (D), трития (Т) и кислорода (18О) в водоочистке, Чистая вода – проблемы и решения, No. 3-4, С. 69-78.
- Мосин, О. В., Игнатов, И. (2011) Современные технологии опреснения морской воды, Энергосбережение, No. 4, С. 14-19.