Water, pH, and Non-Covalent Bonding
Water
Water is essential for life. It covers 2/3 of the earth's surface and every living thing is dependent upon it. The human body is comprised of over 70% water, and it is a major component of many bodily fluids including blood, urine, and saliva. What accounts for the ubiquitous use of water in living systems? If we take a step back and consider the structure of water and compare it to another substance, methane, we can understand the unique properties water possesses that make it well suited for biological systems. Water (H2O) is made up of 2 hydrogen atoms and one oxygen atom, with a total atomic weight of 18 daltons. The structure of the electrons surrounding water is tetrahedral, resembling a pyramid. For comparison, Methane (CH4) is made up of one carbon and 4 hydrogens. Note that methane is similar to water in that it weighs 16 daltons and also has a tetrahedral structure, yet has very different physical properties.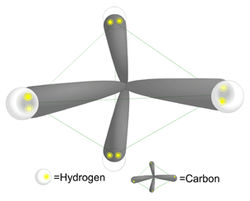
Molecular geometry of methane: pyramidal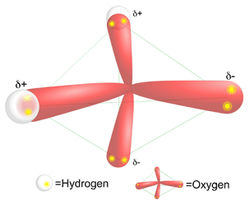
Molecular geometry of water: the orbitals are pyramidal, yet the atoms form a bent shape.
All the vertices on the methane that form the points of a pyramid are occupied by a hydrogen. However, only two vertices in water are occupied with hydrogens. The other two vertices are each occupied by a lone pair of electrons. This fact causes the water molecule to have a bent molecular shape. Oxygen is highly electrophilic (electron loving). This means that even though the oxygen in water is bound to each of the hydrogens by a covalent bond (sharing a pair of electrons), the oxygen "pulls" the shared electrons closer to itself. This unequal sharing of the electrons in the O-H bond in water causes the hydrogens to have a partial positive charge (positive dipole), and the oxygen has a partial negative charge (negative dipole). Water is called a polar molecule because it has a positive side and a negative side, called a dipole moment. In contrast, the carbon in methane shares electrons equally with the 4 hydrogens. Methane's does not have a dipole moment, and in contrast with water is non-polar.
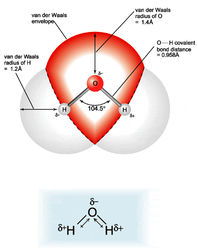
The magnet-like
dipole moment in waterWater molecules, then, have both a partial positive and a partial negative charge. Since opposites attract, this means that water molecules are attracted to each other, like the positive and negative ends of a magnet. Because of these attractive forces, water molecules are in close proximity to one another, making water very dense. Methane molecules do not have a dipole attraction for one another, and therefore and are spaced farther apart. Therefore, despite its similar size and mass to water, methane is much less dense than water. This is the reason that under room temperature situations, water exists as a liquid while methane is a gas. We know that water can be converted to its gaseous phase, steam, but only by applying a lot of energy in the form of heat to disrupt the large attraction of the water molecules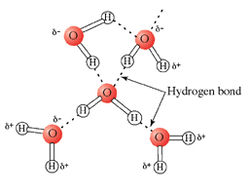
Hydrogen Bonding
Water molecules are bound together through hydrogen bonds. Hydrogen bonds arise when a hydrogen that is covalently bound to either an oxygen, sulfur, or nitrogen atom nears an electrophilic atom, such as oxygen. The electrophilic atom "pulls" the hydrogen closer to itself. The end result is that the hydrogen is now shared (unequally) between the atom to which it is covalently bound and the electrophilic atom to which it is attracted (O-H...O).
Since water is made up solely of hydrogens covalently bound to oxygens and electrophilic oxygens, it is easy to see why there are so many hydrogen bonds in water. The molecules in water are in constant motion and the hydrogen bonds are constantly being broken and reformed. In fact, the average hydrogen bond lasts only a one-trillionth of a second (10-12 s)!
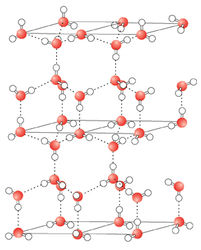
The dynamic nature of hydrogen bonding in waterEach hydrogen bond has an average energy of 20 kJ/mol. This is much less than an O-H covalent bond, which is 460 kJ/mol. Even though an individual hydrogen bond is relatively weak, the large number of hydrogen bonds that exist in water which pull the molecules together give water its special density and phase transition properties. This is much like the story of Gulliver's Travels, when Gulliver awoke on the island of Lilliput to find himself tied down with many tiny strings. Each string was individually weak, but together they were enough to bind Gulliver.
On average, a liquid water molecule will have 3 out of 4 possible hydrogen bonds. This is because the molecules in liquid are in constant motion. In ice, water will form all 4 possible hydrogen bonds because the molecules in a solid are essentially locked in place. Water molecules are further apart from one another in ice than they are in the liquid state. Ice is therefore not as dense as liquid water. This is why icebergs float in the ocean and why ice floats in a beverage. This contrasts greatly from the normal relationship between a compound's solid and liquid forms, with the solid form usually being of a higher density. While the unusual phase density properties of water might not seem too important in biological reactions, it is very important for life on earth. If ice did not float, then in very cold bodies of water ice would sink, removing its insulating effects from the water's surface. Lakes, rivers, and major portions of the oceans thus would freeze completely from the bottom up. The world would be a much different place, and much less suited for life.
Water is a good solvent
The polar nature of water, with its partial positive and partial negative dipole, allows it to dissolve charged molecules (ions) easily. Water is thus an excellent solvent for charged compounds. The positive side of water surrounds negatively charged molecules, and the negatively charged side of water surrounds positively charged molecules.
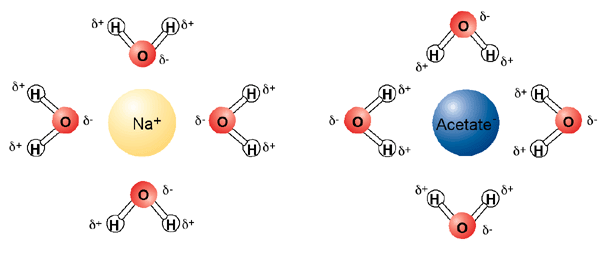
In this way, water makes "solvation shells" around ions. Water can also readily dissolve other polar molecules, even if they are not positively or negatively charged. The solvent properties of water allow for dissolved metals and buffering systems that are very important for the workhorses of life, enzymes. However, the saying "oil and water don't mix" is true--water cannot dissolve oil. This is because oily substances are non-polar. Non-polar substances (which lack dipoles) are also called hydrophobic (water fearing). Hydrophobic substances gather together to exclude water as best they can. This is why you see oil droplets in water. This is also important for the stability and structure of enzymes.
pH
Ionization of Water
Proton hopping in water
Sometimes the hydrogen of one water molecule will "jump" to another water molecule:
H2O + H2O 
This proton hopping is called the ionization of water (an ion is a positively or negatively charged atom or molecule). This ionization creates a H3O+ and a OH- molecule. The H3O+ is often written as simply H+. This is because a H3O+ is just a H+ that jumps from one water molecule to another:
H2O 
So remember, H3O+ = H+
Looking at either of the two chemical equations above, it is important to note that the reverse reaction is also occurring.
How much H+ and OH- exist in water? Very, very little! The ratio of either H+ or OH- to H2O in neutral water is 1:1,000,000,000! Since this is such a small amount of either H+ or OH-, they rarely meet and neutralize each other.
The equilibrium constant, Keq describes the ionization equilibrium of water:
Keq = [H+][OH-]
Because of this relationship it is important to note that if the [H+] goes up then the [OH-] must go down, and vice-versa, for the value for the Keq of water must remain constant. For neutral water, the Keq is 1 x 10-14 M and the concentrations of [H+] and [OH-] are each 1 x 10-7 M . Let's look at that last number without the exponent:
0.0000001 M
This is obviously a very small number. A more manageable way to discuss small numbers such as this is to take the negative logarithm. For the concentration of [H+], this is called the pH. In this case:
-log(0.0000001 M) = 7
The pH of a solution is simply the negative logarithm of [H+]. The pH of a solution describes the acidity of a solution. Acidic solutions are those with a pH of less than 7 and basic solutions have a pH greater than 7. A solution, like H2O, with a pH = 7 is neutral. Similarly, the pOH could be used to describe a solution in terms of its OH- concentration. pOH is the negative logarithm of the OH- concentration. One useful thing to remember is:
pH + pOH = 14.
In the body, the pH of blood is 7.4. This corresponds to a [H+] of about 40 nM. This value can only vary from 37 nM to 43 nM without serious metabolic consequences.
pKa
In living systems, much of the chemistry involves interactions between acids and bases. Acids are H+ donors and bases are H+ acceptors.
HA 
Acid and base reactions are made up of conjugate acid-base pairs. Strong acids are those that readily give up a H+. Strong bases readily accept a H+. Weak acids do not readily give up a H+, but will under the right conditions. A weak base is one that does not easily take up a H+. The conjugate base for a strong acid is a weak base. In contrast, a strong base has a weak acid as its conjugate. Think of a strong acid as a person with an ugly wig. This person can't wait to get rid of this! So now we have a wig (H+) and a person without the wig (the weak base). You can understand that the person without the wig doesn't really want to take it back!
However, what about strong base-weak acid conjugate pairs?
A+ H+ 
Think of it in similar terms: a person in dire need of a wig will grab just about anything. This is our strong base. Now on the right side of the equation we have someone with a wig who really doesn't want to give it up, just as a weak acid does not want to part with its H+.
* We can think of a strong acid or base as having a "flip side", which is its weak base or weak acid conjugate, respectively.
How readily an acid gives up its H+ is expressed by the acid dissociation constant, or Ka:
Ka =
[H+][A-]
[HA]
The pKa is the negative logarithm of the Ka. Strong acids have small pKas. Looking at the above equation, it can be seen that when [A-] = [HA], then Ka = [H+]. Then the pKa = pH. This is the basis for the Henderson-Hasselbalch equation:
pH =
pKa + log
[H+][A-]
[HA]
The Henderson-Hasselbalch
Equation
Titration Curves
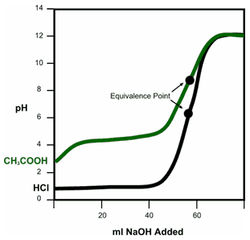
Titration curves of both a strong and weak acid with a strong baseWhen acids or base are added to a solution, the pH changes. The controlled addition of an acid or base to a solution is called a titration. A titration curve can be made by plotting the pH changes against the volume of acid or base added to a solution. Many titration curves are made by plotting pH on the y axis and the volume of base added to the solution on the x axis. For example, pH changes to acetic acid, CH3COOH, and hydrochloric acid, HCl, with 0.1M sodium hydroxide, NaOH, is shown to the left.
Notice the shape of the curves. The CH3COOH curve starts out at a higher pH than the HCl curve. This is because CH3COOH is a weaker acid than HCl. As NaOH is added to the solution of CH3COOH, the pH rises. However, the addition of NaOH to the HCl solution does not significantly change the pH. It takes less NaOH to change the pH of a weak acid solution than it does to change the pH of a strong acid solution. Eventually all of the acid in both solutions will have reacted with base, such that adding more base quickly increases the pH. This is called the equivalence point. This is the point where all the acid has reacted with the base, such that further additions of base quickly raise the pH. The equivalence point for a strong acid-strong base titration is pH = 7. For weak acids-strong base titrations, the equivalence point is pH >7.
Buffers
As shown 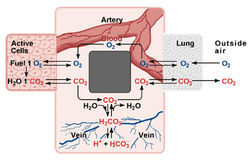
The bicarbonate blood buffering systemabove, the pH of a solution is dependent on the concentration of H+ ions. Addition or removal of H+ ions, then, can greatly affect the pH of a solution. In the body, the pH of cells and extracellular fluids can vary from pH 8 in pancreatic fluid to pH 1 in stomach acids. The average pH of blood is 7.4, and of cells is 7 - 7.3. Although there is great variation in pH between the fluids in the body, there is little variation within each system. For example, blood pH only varies between 7.35 - 7.45 in a healthy individual. Large changes in pH can be life threatening. How does the body maintain a constant blood pH? The body uses a buffer system to withstand changes in pH. Buffers are made up of a mixture of a weak acid with its conjugate base or a weak base with its conjugate acid. Remember that an acid donates a H+. A weak acid does not donate its H+ as easily. Similarly, a weak base will not accept a H+ as well as a strong base.
Buffers maintain pH by binding H+ or OH- ions. This stabilizes changes in pH. The bicarbonate buffer system maintains blood pH near pH 7.4. The carbonic acid, H2CO3, in the blood is in equilibrium with the carbon dioxide (CO2), in the air.
Buffers are most effective in a pH range near its pKa. This is where the titration curve is most shallow, and where the pH is least affected by added acid or base. For example, in the titration curve of phosphoric acid (another blood buffering system):
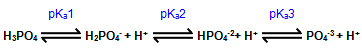
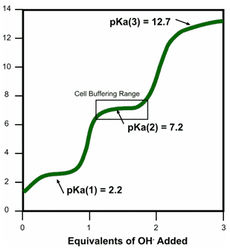
The titration of a polyprotic weak acidThe region in the rectangle is the buffering region of biological importance. Note that the slope of the curve is shallow within one pH unit of the pKa value. This is where the buffering range is most effective, meaning that the buffer is able to resist changes in pH.
Before we move on, let's try some pH Questions
Water, pH, and Non-Covalent Bonding
Non-covalent bonding
Non-covalent bonds are not at strong as covalent bonds, but they are important in the stabilization of molecules. In contrast to covalent bonds, non-covalent bonds do not share electrons. Noncovalent bonds include:
- Electrostatic interactions
- van der Waals forces
- Hydrophobic interactions
- Hydrogen bonds
Electrostatic interactions are formed between positive and negative ions. The bond is non-directional, meaning that the pull of the electrons does not favor one atom over another. An example is NaCl, which is formed between the positively charged Na+ ion and the negatively charged Cl- ion. The average strength of an electrostatic interaction is 15 kilojoules/mol (kJ/mol). The bond strength lessens when the distance between the two ions increases.
van der Waals forces (aka London forces) are weak forces between temporary dipoles. These forces may be attractive or repulsive. They are also non-directional. The average bond energy for van der Waals forces is on the order of 10 kJ/mol.
Hydrophobic interactions result when non-polar molecules are in a polar solvent, e.g. H2O. The non-polar molecules group together to exclude water (hydrophobic means water fearing). By doing so they minimize the surface area in contact with the polar solvent.
A hydrogen bond results when a hydrogen atom that is covalently bound to an electronegative atom (e.g. O, N, S) is shared with another electronegative atom. A hydrogen bond is directional toward the electronegative atom. An example of this is the hydrogen bonds formed in water. Hydrogen bonds are constantly being made and remade. The half-life of a hydrogen bond is about 10 seconds. A hydrogen bond has an energy of 21 kJ/mol.
As stated above, non-covalent bonds are not as strong as covalent bonds, but the additive effect of many non-covalent bonds can stabilize a molecule. Non-covalent bonds are very important in the structure of proteins.
Bond type
Energy (kJ/mol)
Covalent, e.g. C-C
350
Electrostatic
15
van der Waal's
10
Hydrogen
21
Source:
www.wiley.com/college/boyer/0470003790/reviews2011ph_water.htm