Electronic Structure of Ionized Water Clusters
Eric J. Sundstrom, Piotr A. Pieniazek, and Anna I. Krylov
Ionization of liquid water is of great practical interest in the context of atmospheric and biological chemistry. The removal of an electron leads to cascade formation of reactive intermediates that can incur cellular damage. Despite several decades of research the nature of the initially formed state and its immediate dynamics are still poorly understood. We pose a very fundamental question: "What does it mean to ionize water?"
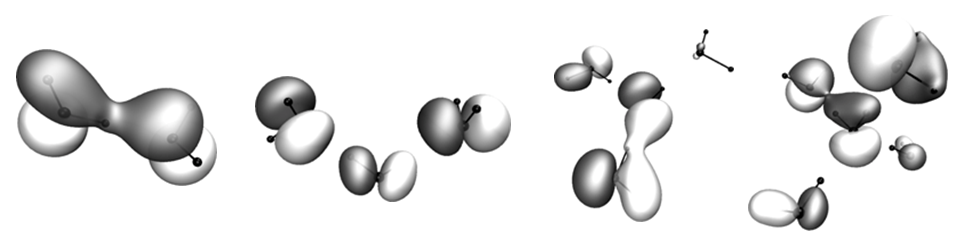
By its nature, liquid water is a disordered system. Thus, a wide range of configurational motifs can be found. To understand this aspect, we studied charge localization in ionized states of a series of water clusters.
The clusters studied were extracted from a simulated box of ice(Ih), using a nearest neighbor algorithm. This extraction led to multiple orientations for each cluster size; representative cluster orientations were chosen and compared with the other orientations of the same size cluster.
The image above, showing a molecular orbital of the pentamer, is rather typical. It shows a large delocalization and signifies a large interaction between the states of individual fragments. In other words, ionization of liquid water creates a hole that spans multiple water molecules. Establishing correlation between cluster states and monomer states in the subject of ongoing research.